Liquids and Solids for the DAT
/Learn key DAT concepts about liquids and solids, plus practice questions and answers
everything you need to know about liquids and solids for the DAT
Table of Contents
Part 1: Introduction to liquids and solids
Part 2: Properties and structures
a) Properties
b) Structures
Part 3: Intermolecular forces
a) Hydrogen bonds
b) Dipole-dipole interactions
c) London dispersion forces
Part 4: Phase changes
a) Isothermal process
b) Phase change diagrams
c) Hess’ Law
Part 5: Vapor pressure
Part 6: High-yield terms
Part 7: Questions and answers
----
Part 1: Introduction to liquids and solids
A foundational understanding of solids and liquids is essential for comprehending why these materials behave the way they do in nature and in chemical reactions. Intermolecular forces play a role in the properties of both solids and liquids. Understanding the unique characteristics of solids and liquids also helps to make sense of phase transitions. As you study this guide, pay attention to any bolded terms, as these are most likely to show up on the DAT. After studying each section, test yourself with DAT-style practice questions at the end.
----
Part 2: Properties and structures
a) Properties
Liquids possess a definite volume without fixed shape, usually conforming to the shape of whatever container they are in. Liquids have multiple properties that are influenced by intermolecular forces. Viscosity, for example, is the measure of a liquid’s resistance to flow due to internal friction. Surface tension is how cohesive the molecules of a liquid’s surface are. Other properties of liquids include boiling point and vapor pressure. These properties, and how they are influenced by intermolecular forces, are explored in the following sections. Solids, on the other hand, have both a definite shape and volume due to the ordered arrangement of particles. Solids cannot be compressed while retaining their structure.
b) Structures
A basic understanding of the structure of liquids and solids will help you succeed on the DAT. In liquids, molecules are closely packed together. This arrangement allows for relative movement and makes liquids more dense than gases. Solid structures, however, vary widely and can be categorized into crystalline and amorphous forms. Crystalline solids exhibit a well-defined, repeating three-dimensional pattern that forms distinct crystals. The arrangement of particles in crystalline solids contributes to their characteristic properties, such as sharp melting points. Crystalline solids can be classified as either ionic, covalent network, metallic, or molecular.
| Ionic | Covalent-network | Metallic | Molecular |
|---|---|---|---|
Crystalline solids are arranged in cubic unit cells that, together, form the crystalline structure known as a crystal lattice. A crystal lattice is the three-dimensional, symmetrical way that atoms are arranged, and the cubic unit cell is the simplest unit that repeats in this structure. There are three types of cubic unit cells, each pictured below. The first type is a simple cubic cell. These cells have 1 atom a piece, with each corner of the cell having an atom that is shared with all neighboring cells. This means that each cell has one-eighth of an atom at all eight of its corners. The second type of cell is known as body-centered cubic and consists of 2 atoms per cell. In addition to the atoms at each corner of the cell, this type of cubic cell has an extra atom located in the center. The final type of cubic cells is face-centered cubic, which has 4 atoms per cell, with half of an atom located at each of the 6 faces of the cell.
FIGURE 1: CUBIC UNIT CELLS OF CRYSTALLINE SOLIDS
Amorphous solids, on the other hand, lack a well-defined structure and exhibit a more random arrangement of particles. Glasses and some plastics are examples of amorphous solids.
----
Part 3: Intermolecular forces
a) Hydrogen bonds
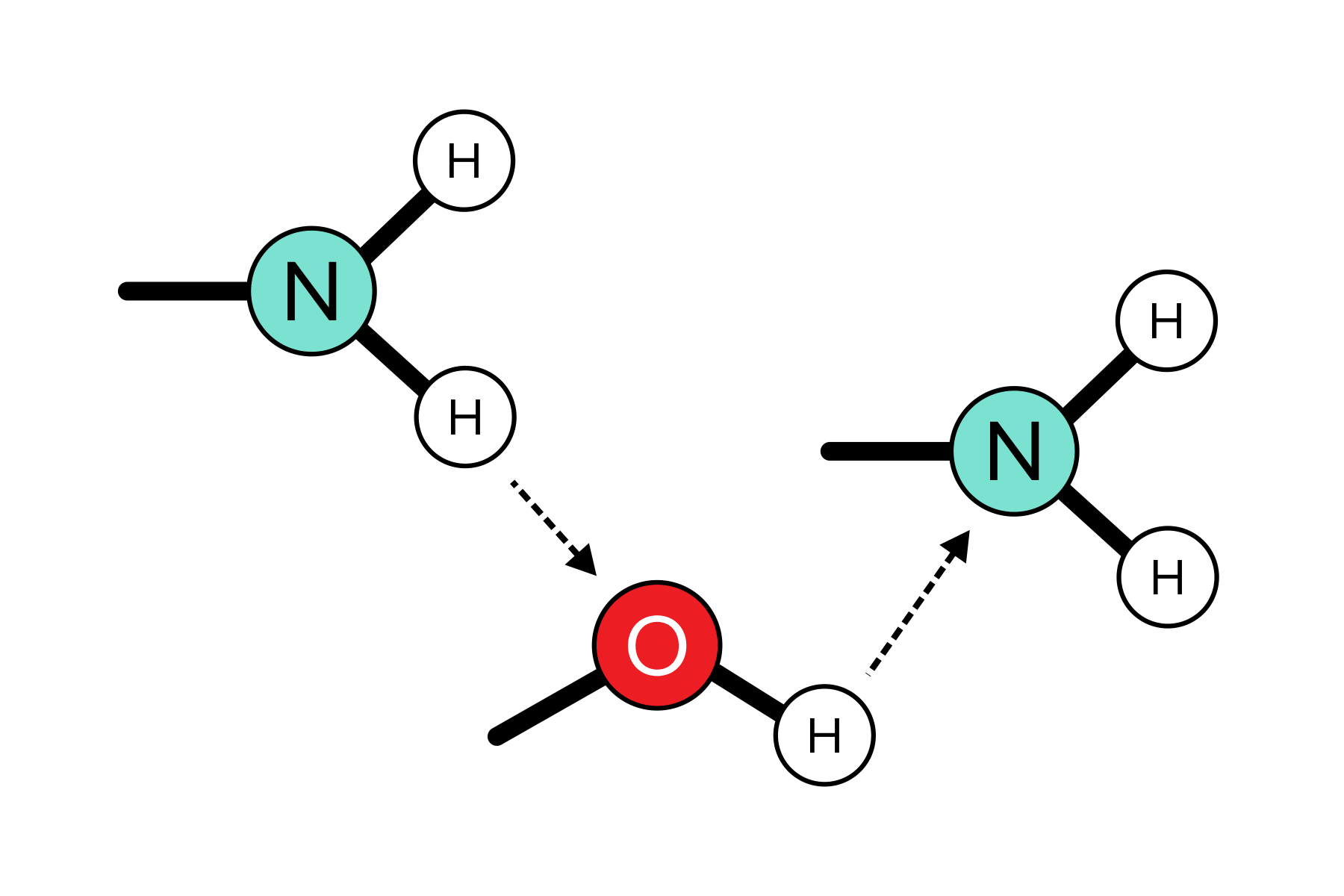
There are three types of intermolecular forces (IMFs)that have an effect on the properties of liquid and solid materials. The first type of IMF is hydrogen bonding. Hydrogen bonds arise when a hydrogen atom forms a bond with an oxygen, fluorine, or nitrogen atom (O, F, or N). The electronegativity of these atoms leads to an uneven sharing of electrons. This uneven sharing results in the hydrogen gaining a partial positive charge, while the O, F, or N atom acquires a partial negative charge. The partial positive charge of the hydrogen on one molecule can interact with the partial negative charges of O, F, or N on another molecule, establishing a robust bond between the two. Notably strong, hydrogen bonds, such as those between water molecules, contribute to the elevated boiling point of water. A substantial amount of heat energy is required to disrupt a hydrogen bond. These bonds are prevalent in various contexts, including nucleotides, water, and organic solvents like alcohol.
FIGURE 2: HYDROGEN BONDING BETWEEN FUNCTIONAL GROUPS ON DIFFERENT MOLECULES
Conventionally, hydrogen bonds are drawn with dashed lines.
b) Dipole-dipole interactions
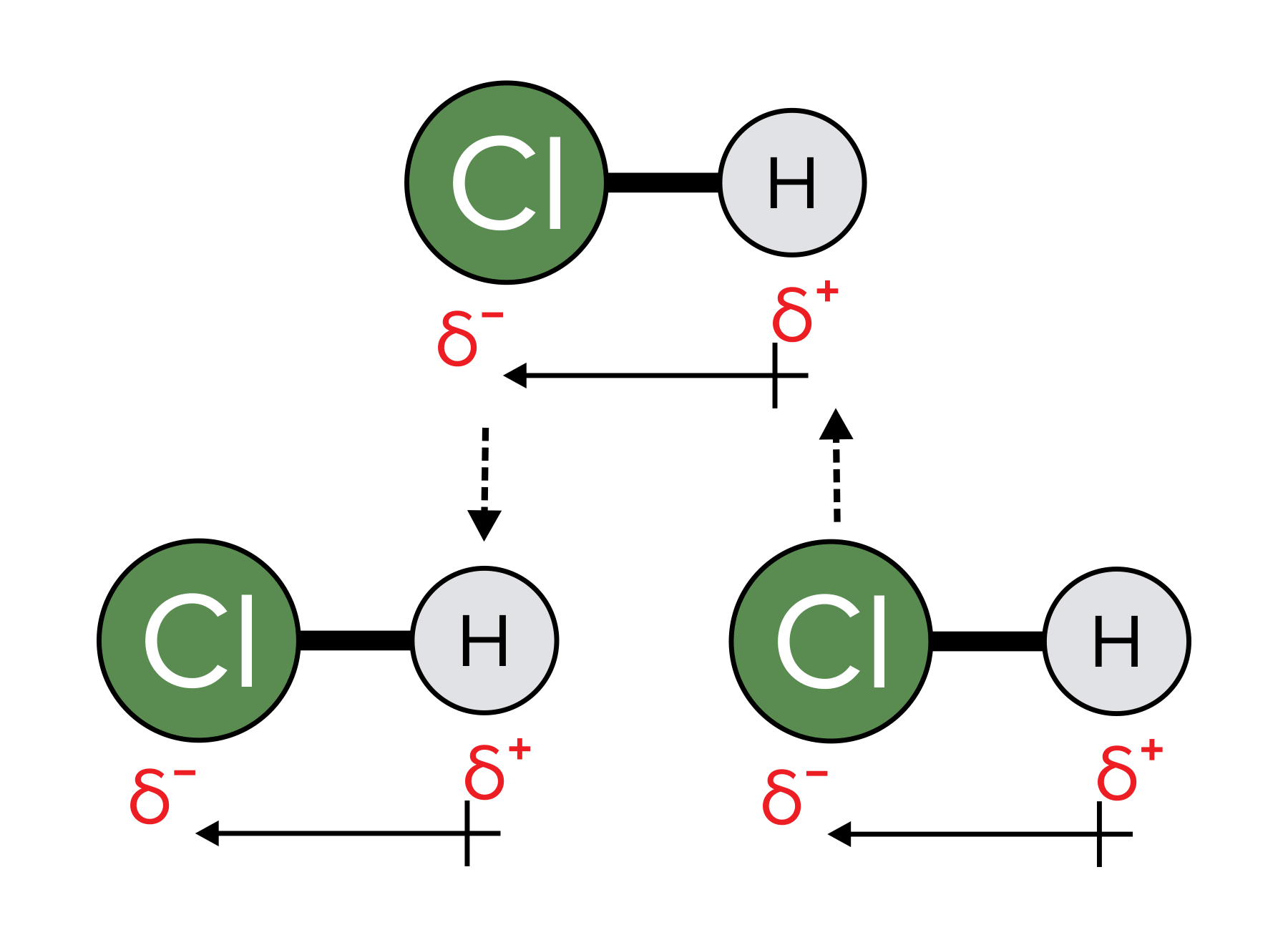
Dipole-dipole interactions emerge as attractive forces when polar molecules orient themselves within a liquid or solid. The partial positive nature of a polar molecule engages with the partial negative aspect of an adjacent molecule. It's essential to remember that this polarity arises from the uneven sharing of electrons between two atoms characterized by differing electronegativities.
FIGURE 3: AN EXAMPLE OF DIPOLE-DIPOLE INTERACTIONS BETWEEN HYDROCHLORIC ACID. NOTE THAT THE SYMBOL INDICATES A “PARTIAL” POSITIVE OR NEGATIVE CHARGE RATHER THAN A COMPLETELY POSITIVE OR NEGATIVE CHARGE.
Hydrogen bonding represents a distinctive type of dipole-dipole interaction, distinguished by its exceptional stability arising from the substantial electronegativity contrast between hydrogen and atoms such as N, O, or F. In comparison, dipole-dipole interactions may exhibit less stability, attributable to a smaller electronegativity difference and a more balanced sharing of electrons.
c) London dispersion forces
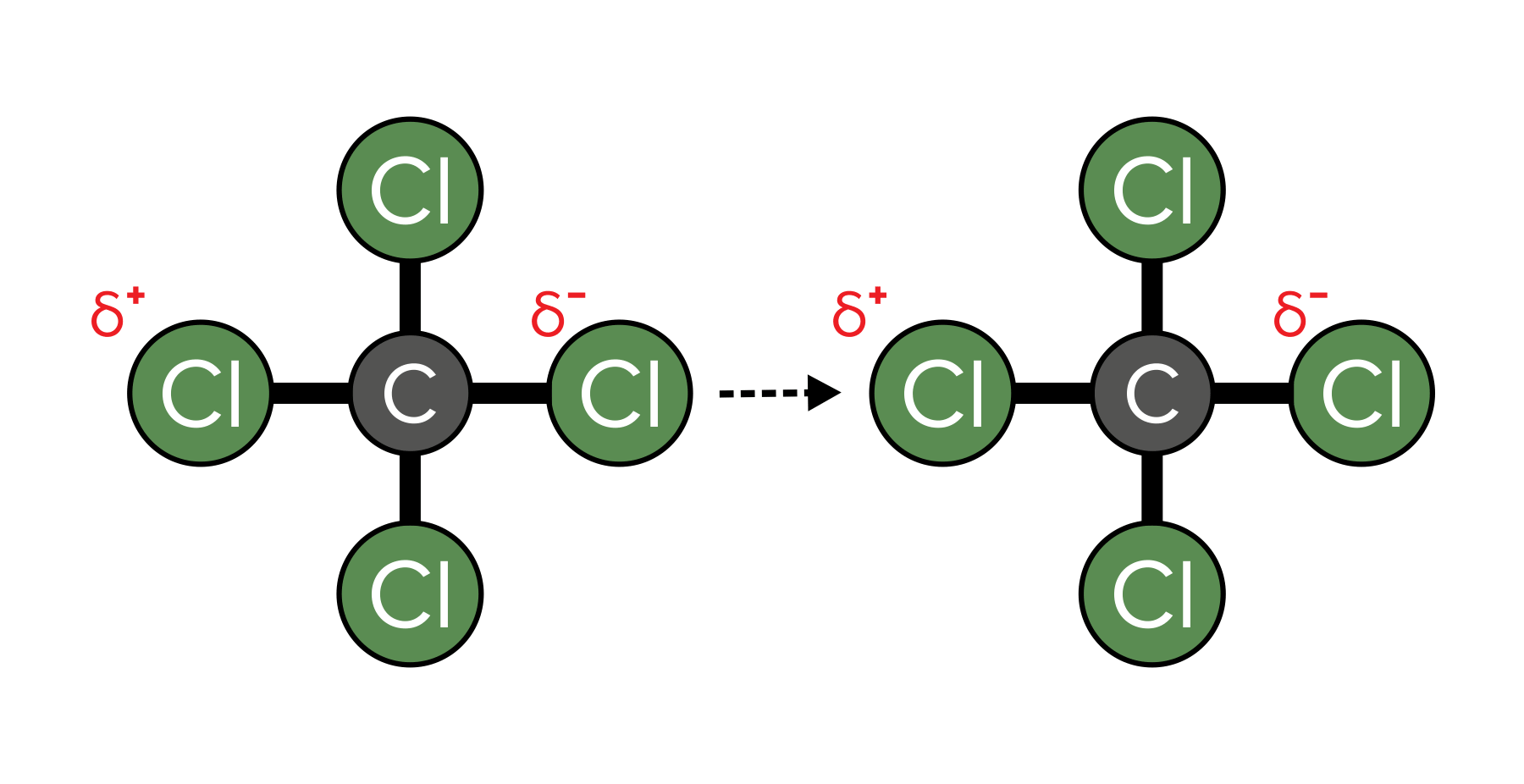
London dispersion forces, also referred to as van der Waals interactions, arise from the uneven distribution of electrons within a molecule. Despite our perception of electron distribution as uniform, random movements can induce polarization in a molecule. Briefly, electrons may gather on one side, resulting in a temporary partial negative charge. This charge induces a corresponding temporary partial positive charge in a neighboring molecule and creates a chain of temporary partial charges that generate an attractive force known as London dispersion forces. These forces, being the weakest and most fleeting, rely on transient and uneven electron distribution. Additionally, they are contingent upon the distance between molecules, with closer proximity enhancing the magnitude of van der Waals interactions. Despite their weakness, these forces play a pivotal role in maintaining molecules in their liquid and solid phases.
FIGURE 4: AN EXAMPLE OF VAN DER WAALS ATTRACTIONS. NOTICE HOW TEMPORARY CHANGES IN ELECTRON DISTRIBUTION CAUSE PARTIAL CHARGES, WHICH CREATE INTERMOLECULAR ATTRACTIONS.